Cranfield’s New products – Polymers for Pharmaceuticals
05/10/2021
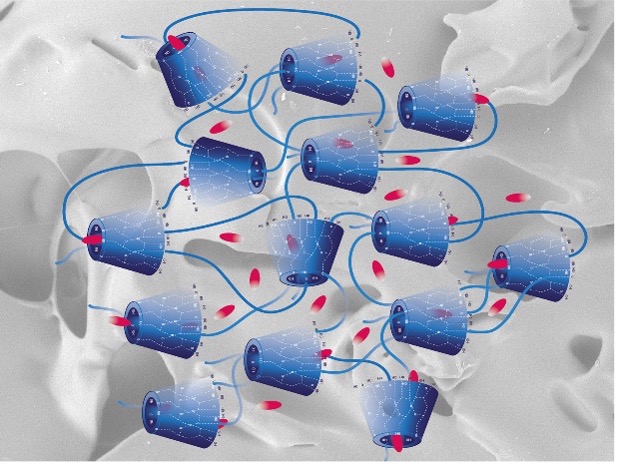
Figure 1 – Schematic representation of cross-linked β-cyclodextrin and entrapped molecules. SEM image of the amorphous polymer as background (15.0kVx50 BSE-3D 60Pa, 1 mm). Image by C. Pratchett, Cranfield University.
Polymers are very important constituents for therapeutic applications as well as the largest and versatile class of biomaterials [1,2]. Synthetic polymers can be designed and synthesised to have a broad variety of structures and appropriate physical and chemical properties; they are used in a wide range of biomedical applications as diverse as tissue engineering, drug delivery, therapeutics, diagnostics, and other applications.
Since the last century, methods of polymer synthesis, processing, and characterisation have been developing rapidly, which bring both challenges and opportunities to design novel polymeric biomaterials and to understand the biological interactions between biological systems and polymeric materials [3].
Polymers possess significant potential since their chemical flexibility gives rise to materials with diverse physical and mechanical property diversity. Degradable polymers are of utmost interest since these biomaterials are able to be broken down and excreted or resorbed without removal or surgical revision [4].
Special attention is paid by scientists and engineers on polymers that occur in nature and can be extracted and modified. Polysaccharides are used as biomaterials and become much more common as new biological functions are identified for these materials. They are natural polymers composed of monosaccharide units joined together by glycosidic linkages. Furthermore, the array of materials that can be investigated has increased due to new synthetic routes that have been developed for modifying polysaccharides. Their biodegradability, processability, and bioactivity make polysaccharides a very promising natural biomaterials [4,5].
Cyclodextrins (CDs), a family of cyclic polysacchararides produced from starch by enzymatic conversion have been widely investigated as a drug delivery vehicle due to their ability to envelop guest molecules [6]. The three best-known CDs are α-cyclodextrin (αCD), β-cyclodextrin (βCD), and γ-cyclodextrin (γCD), which are toroidal macrocycles of six, seven and eight glucopyranose units, respectively. CDs have been chemically modified to adjust their solubility, bioavailability and inclusion complex stability. There is great interest in these compounds, reflecting their ability to form supramolecular structures with exploitable guest-host interactions [6].
Semi-synthetic polymeric systems based on cyclodextrins are manufactured for the first time at Cranfield University in the UK [7-11] from renewable resources and by “green chemistry” processes. The Green Chemistry concept applies throughout the entire life cycle of a chemical product, from its design and manufacturing to its use and final disposal [12] and relates to the chemicals, process and products.
The properties of crystalline β-cyclodextrin have been modified by cross-linking in water with a family of non-toxic diepoxides having variable length of polyethylene glycol spacers. Two different polymeric systems with large hydrodynamic volume were obtained:
- Soluble cross-linked polymers;
- Hydrogels [13,14].
The reaction parameters were tuned to obtain water and alcohol soluble products, which are malleable and suitable for processing and can also be easily further modified by subsequent substitution reactions. The thermal and thermo-mechanical properties of the cross-linked products are directly dependent on the content and length of the polyethylene spacer. The glass transition temperature (Tg) as low as -30 °C, which extends the operative range at which the cross-linked cyclodextrins can be processed as soft polymers. The cross-linked material with the longer polyethylene spacer and its nitrated analogue can restore their integrity by self-healing. The high hydrodynamic volume of this polymer in solution enhances the solubilisation and complexation of small molecules such as curcumin [15]. Further cross-linking reaction in the oven produced insoluble network structures with good physical integrity and important swelling power up to 200%.
The new polymeric cyclodextrin-based systems developed through green chemistry are promising passive and/or active ingredients for a variety of biomedical applications, just to mention some:
- Drug delivery systems
- Heart diseases
- Vaccine adjuvants
- Toxin’s absorption
- Coatings.
References
- E. Mathiowitz, Encyclopedia of Controlled Drug Delivery (2-Volume Set), E. Mathiowitz ed. 1999, ISBN: 0-471-14828-8.
- M.F Maitz, Biosurface and Biotribology 2015, 1, 161, https://doi.org/10.1016/j.bsbt.2015.08.002.
- R. Blagoeva, A. Nedev, Bioautomation 2006, 4, 80. ISSN 1312–451X.
- B. D. Ulery, L. S. Nair,C. T. Laurencin, J Polym Sci B Polym Phys. 2011, 49, 832. DOI: 10.1002/polb.22259.
- J. Kost, Encyclopedia of Controlled Drug Delivery (2-Volume Set), E. Mathiowitz ed. 1999, p. 445. ISBN: 0-471-14828-8.
- C. Palomino-Durand, M. Lopez, F. Cazaux, B. Martel, N. Blanchemain, F. Chai, Polymers (Basel, Switzerland) 2019, 11. DOI:10.3390/polym11020214.
- F. Luppi, H. Cavaye, E. Dossi, Propellants, Explosives, Pyrotechnics 2018, 43, 1023. https://doi.org/10.1002/prep.201800137.
- F. Luppi, G. Kister, M. Carpenter, E. Dossi, Polymer Testing 2019, 73, 338. https://doi.org/10.1016/j.polymertesting.2018.11.034.
- F. Luppi, N. Mai, G. Kister, P.P. Gill, S.E. Gaulter, C. Stennett, E. Dossi, Chemistry A European Journal 2019, Early view. https://doi.org/10.1002/chem.201903945.
- E. Dossi, G. Kister, M.E. Bolton, Oral contribution at 6th European Conference on Cyclodextrins 2019, Santiago de Compostella, 2-4 October.
- E. Dossi, M.E. Bolton, G. Kister, A. Afsar, 2021, Accepted for publishing (July 2021) in ChemistrySelect.
- United States Environmental Protection Agency. Basics of green chemistry 2017. Available at: https://www.epa.gov/greenchemistry/basics-green-chemistry (Accessed: 6 June 2020)
- A. M. Lowman, N. A. Peppas, “Encyclopedia of Controlled Drug Delivery” (2-Volume Set) E. Mathiowitz ed. 1999, p 397. ISBN: 0-471-14828-8.
- N. A. Peppas, J. Zach Hilt, A. Khademhosseini, Robert Langer, Adv. Mater. 2006, 18, 1345. Doi: 10.1002/adma.200501612.
- Á. Haimhoffer, E. Dossi, V. Fejes, J. Váradi, G. Vasvári, I. Bácskay, F. Fenyvesi, 2021, in preparation for submission in Pharmaceutics.
Categories & Tags:
Leave a comment on this post:
You might also like…
Keren Tuv: My Cranfield experience studying Renewable Energy
Hello, my name is Keren, I am from London, UK, and I am studying Renewable Energy MSc. My journey to discovering Cranfield University began when I first decided to return to academia to pursue ...
3D Metal Manufacturing in space: A look into the future
David Rico Sierra, Research Fellow in Additive Manufacturing, was recently involved in an exciting project to manufacture parts using 3D printers in space. Here he reflects on his time working with Airbus in Toulouse… ...
A Legacy of Courage: From India to Britain, Three Generations Find Their Home
My story begins with my grandfather, who plucked up the courage to travel aboard at the age of 22 and start a new life in the UK. I don’t think he would have thought that ...
Cranfield to JLR: mastering mechatronics for a dream career
My name is Jerin Tom, and in 2023 I graduated from Cranfield with an MSc in Automotive Mechatronics. Originally from India, I've always been fascinated by the world of automobiles. Why Cranfield and the ...
Bringing the vision of advanced air mobility closer to reality
Experts at Cranfield University led by Professor Antonios Tsourdos, Head of the Autonomous and Cyber-Physical Systems Centre, are part of the Air Mobility Ecosystem Consortium (AMEC), which aims to demonstrate the commercial and operational ...
Using grey literature in your research: A short guide
As you research and write your thesis, you might come across, or be looking for, ‘grey literature’. This is quite simply material that is either unpublished, or published but not in a commercial form. Types ...